1. Introduction
Malaria mortality has dropped worldwide due to the worldwide malaria eradication program and the use of artemisinin-based combination therapy and some medications. However, the prevalence of Plasmodium falciparum strains that are resistant to standard drugs such as chloroquine has increased in many parts of the world. In recent years, one of the main causes of death from malaria has been reported to be increased drug resistance; hence, scientists are looking for new drugs to control the disease.
With the advancement of medical science and related sciences, many studies have been conducted on the chemical properties of various toxins. Today, snake venom can be used to prepare various drugs, serums and vaccines. In recent years, researchers have been investigated the properties of snake venoms for increasing the drug resistance to Plasmodium falciparum. Their results have shown that the venom of Bothrops asper snake and South American rattlesnake have cell penetrating, antifungal and antiparasitic properties. Iranian cobra (Naja Naja Oxiana) is a snake from the family Elapidae that is found in large areas in northeastern Iran. The aim of this study was to investigate the anti-malaria effect (in the ring stage) of the active fraction isolated from Iranian cobra venom by determining the parasitic load using Real-time PCR.
2. Materials and Methods
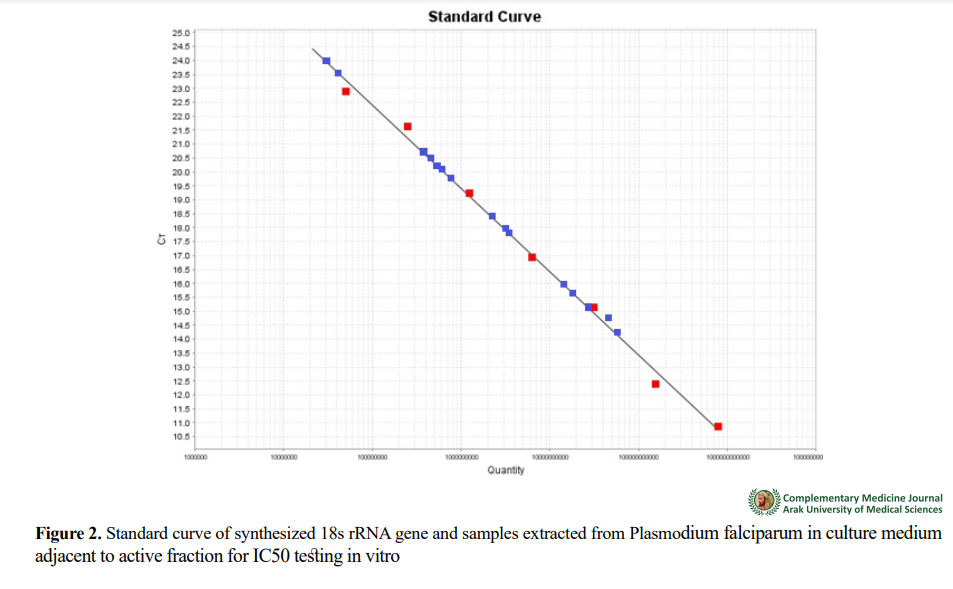
After determining the effective dose of active fraction, the materials were first mixed slowly and on ice with a sampler in two vials of 0.2 cc. For all primers used in the Real-time PCR, 10% stocks were prepared from the initial stock of 100 µl and then the required volumes of primers were added to 15 µL of PCR reaction. There were 15 ng samples in each µL of DNA. After a spin of 5 seconds, we finally transferred the strips to the Real-time PCR device to operate under the determined settings. In this method, a DNA sample with a specific concentration was used to draw a standard curve. The standard DNA concentration was determined with a 260-nm spectrophotometer and then converted to the number of copies based on the molecular weight of the sample. The 18s rRNA gene was sequenced and then sent to Takapouzist company for synthesis. After synthesis, serial dilution was prepared from the standard samples and placed in real-time PCR device with the target sample. Using the Ct (Threshold Cycle) value for each dilution, we drawed a curve in which X axis showed the dilution or the number of copies of the gene and Y represented the expected Ct value. The length of the gene sequence was 234 bp. When the lyophilized state was liquefied by adding 100 µ l of injected distilled water, its concentration was measured with a nanodrop.
3. Results
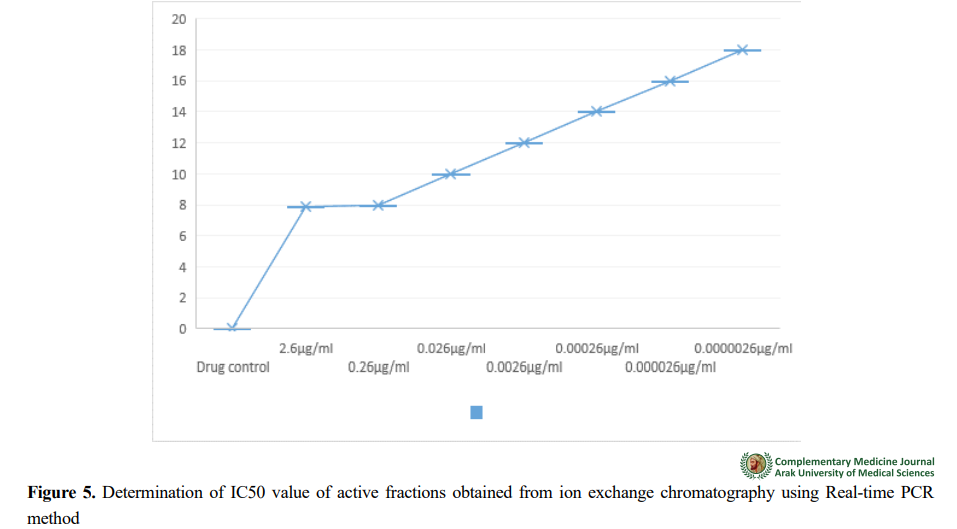
For quantitative analysis of gene expression, information obtained from 18 s rRNA gene expression was synthesized and cloned with the results of the standard curve of 18s rRNA gene and normalized in PUC57. With the help of amplification diagrams, the CT value of the samples was determined, and the presence of non-specific products and primer dimers was determined with the help of melting curve. Figures 1, 2, 3, 4, and 5 plot the standard, amplification, and melting curves of the 18s rRNA gene. They showed the specificity of real-time PCR conditions for this gene.
.PNG)


4. Conclusion
The obtained results showed that the anti-Plasmodium effect of the active fraction of snake venom is quite apparent. These promising results motivate further research in this area; Therefore, according to the obtained results, it will not be far-fetched to develop new effective antimalarial drugs after systematic research in this field.
Ethical Considerations
Compliance with ethical guidelines
This research observed the ethical principles according to Declaration of Helsinki, and obtained its ethical approval from Iran University of Medical Sciences (code: IR.IUMS.REC1395.9223651202).
Funding
This research was supported by the research project (No. IR.IUMS.REC1395.9223651202), Funded by the Iran University of Medical Sciences.
Authors' contributions
All authors contributed equally in preparing this article.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the Iran University of Medical Sciences and Pasteur Institute of Iran for funding and cooperation in conducting specialized tests.
- Chirebvu E, Chimbari MJ, Ngwenya BN. Assessment of risk factors associated with malaria transmission in tubu village, northern botswana.Malaria Research and Treatment. 2014; 2014:403069. [DOI:10.1155/2014/403069] [PMID] [PMCID]
- Sant’Ana CD, Ticli FK, Oliveira LL, Giglio JR, Rechia CG, Fuly AL, et al. BjussuSP-I: A new thrombin-like enzyme isolated from Bothrops jararacussu snake venom. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology. 2008; 151(3):443-54. [DOI:10.1016/j.cbpa.2007.02.036] [PMID]
- Utkin YN. Animal venom studies: Current benefits and future developments. World Journal of Biological Chemistry. 2015; 6(2):28-33. [DOI:10.4331/wjbc.v6.i2.28] [PMID] [PMCID]
- Liu CC, Yang H, Zhang LL, Zhang Q, Chen B, Wang Y. Biotoxins for cancer therapy. Asian Pacific Journal of Cancer Prevention 2014; 15(12):4753-8. [DOI:10.7314/APJCP.2014.15.12.4753] [PMID]
- Dhananjaya BL, Sivashankari PR. Snake venom derived molecules in tumor angiogenesis and its application in cancer therapy; An overview. Current Topics in Medicinal Chemistry. 2015; 15(7):649-57. [DOI:10.2174/1568026615666150225113402] [PMID]
- Malleswari M, Josthna P, Doss PJ. Orally Administered Venom of Naja Naja Alters Protein Metabolic Profiles in the Liver of Albino Rats. International Journal Of Life Sciences Biotechnology And Pharma Research. 2015; 4(1):10-6. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.676.7515&rep=rep1&type=pdf
- Heydarian P, Nateghpour M, Mazhari N, Motevalli Haghi A, Farivar L, Souri E, et al. Evaluation of Effectiveness of Ethanolic Extract of Curcuma longa, discretely and in Combination with Chloroquine against Chloroquine-Sensitive Strain of Plasmodium berghei. Herbal Medicines Journal. 2018; 3(4):133-8. http://eprints.lums.ac.ir/id/eprint/1961
- Garedaghi Y, Khaki A. Evaluation of the effectiveness of ethanolic extract of solanum surattense against plasmodium berghei in comparison with chloroquine in sourian mice using in vivo tests. Crescent Journal of Medical and Biological Sciences. 2014; 1(3):76-9. http://www.cjmb.org/text.php?id=113
- Massimine KM, McIntosh MT, Doan LT, Atreya CE, Gromer S, Sirawaraporn W, et al. Eosin B as a novel antimalarial agent for drug-resistant Plasmodium falciparum. Antimicrobial Agents and Chemotherapy. 2006; 50(9):3132-41. [DOI:10.1128/AAC.00621-06] [PMID] [PMCID]
- Ebrahim K, Vatanpour H, Zare A, Shirazi FH, Nakhjavani M. Anticancer activity a of Caspian Cobra (Naja naja oxiana) snake venom in human cancer cell lines via induction of apoptosis. Iranian Journal of Pharmaceutical Research. 2016; 15(Suppl):101-12. [PMID] [PMCID]
- Castillo JC, Vargas LJ, Segura C, Gutiérrez JM, Pérez JC. In vitro antiplasmodial activity of phospholipases A2 and a phospholipase homologue isolated from the venom of the snake Bothrops asper. Toxins. 2012; 4(12):1500-16. [DOI:10.3390/toxins4121500] [PMID] [PMCID]
- El Chamy Maluf S, Dal Mas C, Oliveira EB, Melo PM, Carmona AK, Gazarini ML, et al. Inhibition of malaria parasite Plasmodium falciparum development by crotamine, a cell penetrating peptide from the snake venom. Peptides. 2016; 78:11-6. [DOI:10.1016/j.peptides.2016.01.013] [PMID]
- Hajialiani F, Elmi T, Mohamadi M, Sadeghi S, Shahbazzadeh D, Ghaffarifar F, et al, Analysis of the active fraction of Iranian Naja naja oxiana snake venom on the metabolite profiles of the malaria parasite by 1HNMR in vitro. Iranian Journal of Basic Medical Sciences. 2020; 23(4):534-43. [DOI:10.1128/JCM.43.5.2435-2440.2005]
- Mangold KA, Manson RU, Koay ES, Stephens L, Regner M, Thomson RB Jr, et al. Real-time PCR for detection and identification of Plasmodium spp. Journal of Clinical Microbiology. 2005; 43(5):2435-40.[DOI:10.1128/JCM.43.5.2435-2440.2005] [PMID] [PMCID]
- Taylor BJ, Martin KA, Arango E, Agudelo OM, Maestre A, Yanow SK. Real-time PCR detection of Plasmodium directly from whole blood and filter paper samples. Malaria Journal. 2011; 10:244.[DOI:10.1186/1475-2875-10-244] [PMID] [PMCID]
- Ebrahimzadeh A, Fouladi B, Fazaeli A. High rate of detection of mixed infections of Plasmodium vivax and Plasmodium falciparum in South-East of Iran, using nested PCR. Parasitology International. 2007; 56(1):61-4. [DOI:10.1016/j.parint.2006.12.001] [PMID]
- Taylor SM, Juliano JJ, Trottman PA, Griffin JB, Landis SH, Kitsa P, et al. High-throughput pooling and Real-time PCR-based strategy for malaria detection. Journal of Clinical Microbiology. 2010; 48(2):512-9. [DOI:10.1128/JCM.01800-09] [PMID] [PMCID]
- Guillaume C, Deregnaucourt C, Clavey V, Schrével J. Anti-Plasmodium properties of group IA, IB, IIA and III secreted phospholipases A2 are serum-dependent. Toxicon. 2004; 43(3):311-8. [DOI:10.1016/j.toxicon.2004.01.006] [PMID]
- Parvazi S, Sadeghi S, Azadi M, Mohammadi M, Arjmand M, Vahabi F, et al. The effect of aqueous extract of cinnamon on the metabolome of Plasmodium falciparum using 1HNMR spectroscopy. Journal of Tropical Medicine. 2016; 2016:3174841.[DOI:10.1155/2016/3174841] [PMID] [PMCID]
- Zakeri S, Mamaghani S, Mehrizi A, Shahsavari Z, Raeisi A, Arshi S, et al. Molecular evidence of mixed P. vivax and P. falciparum infections in northern Islamic Republic of Iran. Eastern Mediterranean health journal. 2004; 10(3):336-42. https://apps.who.int/iris/handle/10665/119418


 , Sedigheh Sadeghi2
, Sedigheh Sadeghi2 

 , Delavar Shahbazzadeh3
, Delavar Shahbazzadeh3 

 , Fatemeh Tabatabaie
, Fatemeh Tabatabaie 

 4, Zahra Zamani2
4, Zahra Zamani2 


.PNG)




