Introduction
Diabetes is one of the most common endocrine disorders [
1]. The number of patients with diabetes is increasing; it is estimated that it will reach 700 million people by 2045 [
2]. High blood sugar in diabetes is associated with complications such as osteoporosis, decreased muscle quality, and cardiovascular diseases [
3]. Growing evidence shows that arginase enzyme plays a key role in the complications of diabetes, and modulating this enzyme can be a suitable treatment to reduce the complications of the disease. Arginase is an important enzyme in the urea cycle to convert L-arginine to ornithine and urea. The increase in arginase enzyme expression under high blood sugar conditions may lead to disturbances in nitric oxide production and endothelial function [
5]. Recently, it has been reported that activated p38 increases the expression of arginase in diabetic rats. In addition, by using MAP-kinase inhibitors, it has been shown that p38 MAPK is an upstream activator in a signaling cascade that leads to the increase of arginase in high blood sugar conditions [
7].
Studies have shown that cinnamon enhances the action of insulin and activates the nitric oxide production pathway [
14]. On the other hand, aerobic exercise reduces the basal level of p38 activation [
20]; in this way, it may prevent the increase of arginase in diabetes. Considering the role of arginase enzyme in high blood sugar and the effect of exercise and cinnamon extract on the upstream protein of these enzymes, the present study aims to assess whether aerobic exercise combined with cinnamon extract can modulate arginase enzyme in diabetes.
Methods
In this study, 35 male Wistar rats aged 8 weeks, after 7 days of adaptation to the new environment, induction of diabetes and adaptation to exercise on a treadmill, were randomly divided into five groups: control (C), diabetic (D), diabetic + supplement (DS), diabetic + exercise (DE) and diabetic + exercise + supplement (DES) based on the weight. Mice became diabetic using nicotinamide and streptozotocin [
21]. The aerobic training program was started in a progressive manner. This exercise program was based on Chia et al.’s study and had a moderate intensity [
23]. The exercise groups performed running on treadmills at 5 sessions a week for 8 weeks. The rats in DS and DES groups received 200 mg/kg hydroalcoholic cinnamon extract daily by gavage for 8 weeks, and those in DE and D groups received placebo (water) during this period [
24]. All rats were anesthetized by intraperitoneal injection of ketamine 48 hours after the last training session. Then, the soleus muscle was isolated and immediately kept at -80° C for assessment. The values of arginase and p38 MAPK were measured by ELISA kits according to the instructions of the manufacturer. Data are reported as Mean±SD. The results of Shapiro-Wilk test showed that the data had a normal distribution. For between-group comparisons, one-way analysis of variance was used, followed by Tukey’s post hoc test. Pearson correlation test was used to assess the relationship between the study variables, considering a significance level of p<0.05.
Results
The mean weight of rats at baseline was not significantly different between the study groups (F=0.948, p=0.45), but after the intervention, the weight of all diabetic rats decreased significantly compared to group C (p=0.000). In addition, the weight of rats in the DS (p=0.02) and DES (p=0.0005) groups had a significant increase compared to group D. Blood glucose changes showed that the blood glucose concentration of diabetic groups was significantly higher than that of group C (p=0.000). In the post-test phase, the glucose concentration of groups DE (p=0.00005), DS (p=0.00005) and DES (p=0.0005) was significantly lower compared to that of group D. Moreover, the blood glucose concentration of DS and DES groups were significantly different (p=0.025), but there was no significant difference between groups DS and DE (p=0.93) and groups DE and DES (p=0.155). Arginase protein levels in groups D (p=0.000), DS (p=0.000) and DE (p=0.000) were significantly higher than in group C. Arginase protein level in the DES group was significantly lower than in groups D (p=0.000), DS (p=0.000) and DE (p=0.000) (
Figure 1).
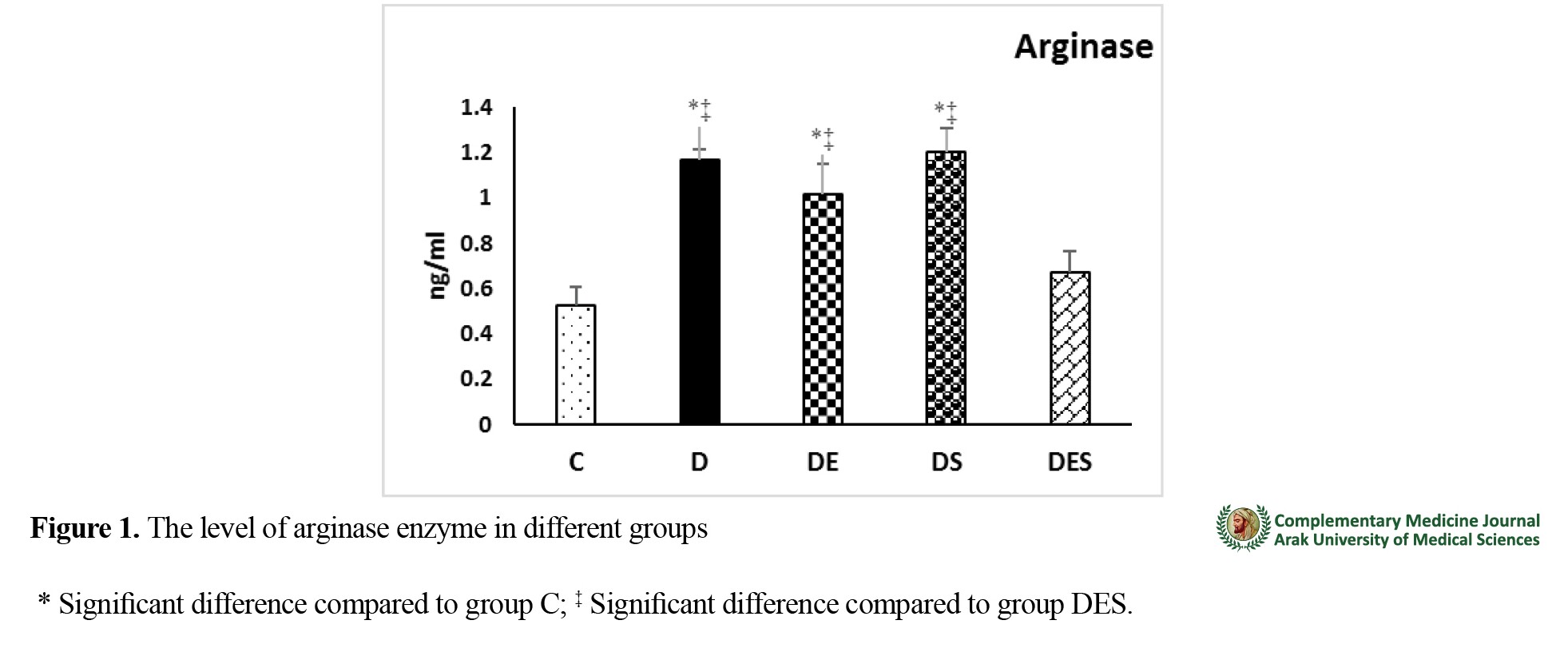
Changes in p38 MAPK protein level showed no significant difference among the groups (F=2.482, p=0.07). There was a significant positive correlation between arginase and blood glucose levels (r=0.547, p=0.000). However, there was no correlation between p38 MAPK and blood glucose levels (r=0.096, p=0.556) and between p38 MAPK and arginase protein levels (r=0.213, p=0.187) .
Discussion
The combination of aerobic exercise and cinnamon extract supplementation can reduce the level of arginase enzyme. Therefore, considering that many complications of diabetes are related to the increase of arginase enzyme, this interventional method can help modulate the complications of diabetes.
Ethical Considerations
Compliance with ethical guidelines
This study obtained its ethical approval from Kurdistan University of Medical Sciences (Code: IR.MUK.REC.1398.5008).
Funding
This study was extracted from a PhD. dissertation of first author in Department of Sport Sciences, Faculty of Literature & Human Sciences, Lorestan University, Khorramabad.
Authors' contributions
Conceptualization, methodology, investigation, resources, writing-original draft preparation, writing-review & editing, visualization, supervision, project administration: Ehsan Hoseininejad and Vahid Valipour Dehnou; Conceptualization, methodology, writing – review & editing, visualization, supervision: Ehsan Ghahramanlou; Software, validation, formal analysis, investigation, resources, data curation, writing – review & editing, visualization: Ali Gorzi.
Conflicts of interest
The authors declared no conflict of interest.
References
- Parastesh M, Heidarianpour A, Sadegh M. Investigating the effects of endurance, resistance and combined training on reproductive hormones and sperm parameters of streptozotocin-nicotinamide diabetic male rats. Journal of Diabetes and Metabolic Disorders. 2019; 18(2):273-9. [DOI:10.1007/s40200-018-0380-4] [PMID] [PMCID]
- Demir S, Nawroth PP, Herzig S, Ekim Üstünel B. Emerging targets in type 2 diabetes and diabetic complications. Advanced Science. 2021; 8(18):2100275. [DOI:10.1002/advs.202100275] [PMID] [PMCID]
- Sangani R, Naime M, Zakhary I, Ahmad S, Chutkan N, Zhu A, et al. Regulation of vitamin C transporter in the type 1 diabetic mouse bone and bone marrow. Experimental and Molecular Pathology. 2013; 95(3):298-306. [DOI:10.1016/j.yexmp.2013.08.007] [PMID]
- Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circulation Research. 2008; 102(1):95-102. [DOI:10.1161/CIRCRESAHA.107.155028] [PMID] [PMCID]
- Kovamees O, Shemyakin A, Eriksson M, Angelin B, Pernow J. Arginase inhibition improves endothelial function in patients with familial hypercholesterolaemia irrespective of their cholesterol levels. Journal of internal Medicine. 2016; 279(5):477-84. [DOI:10.1111/joim.12461] [PMID]
- Pandya CD, Lee B, Toque HA, Mendhe B, Bragg RT, Pandya B, et al. Age-dependent oxidative stress elevates arginase 1 and uncoupled nitric oxide synthesis in skeletal muscle of aged mice. Oxidative Medicine and Cellular Longevity. 2019; 2019:1704650. [DOI:10.1155/2019/1704650] [PMID] [PMCID]
- Mazrouei S. Regulation of MAP kinase-mediated endothelial dysfunction in hyperglycemia via arginase I and eNOS dysregulation. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2019; 1866(9):1398-411. [DOI:10.1016/j.bbamcr.2019.05.004] [PMID]
- Shosha E, Xu Z, Narayanan SP, Lemtalsi T, Fouda AY, Rojas M, et al. Mechanisms of diabetes-induced endothelial cell senescence: Role of arginase 1. International Journal of Molecular Sciences. 2018; 19(4):1215. [DOI:10.3390/ijms19041215] [PMID] [PMCID]
- Ebadi M. Pharmacodynamic basis of herbal medicine. Florida: CRC press; 2006. [DOI:10.1201/9781420006452]
- Hlebowicz J, Darwiche G, Björgell O, Almér LO. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. The American Journal of Clinical Nutrition. 2007; 85(6):1552-6. [DOI:10.1093/ajcn/85.6.1552] [PMID]
- Khan A, Safdar M, Khan MMA, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003; 26(12):3215-8. [DOI:10.2337/diacare.26.12.3215] [PMID]
- Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in Non-insulin-dependent type 2 diabetes. Diabetes Care. 2007; 30(9):2236-7. [DOI:10.2337/dc07-0098] [PMID]
- Khadem Haghighian H, Farsad Naimi A, Pourghassem Gargari B, Ali-Asgharzadeh A, Nemati A. [Effect of cinnamon on glycemic control and insulin resistance in type II diabetes patients: A randomized clinical trial (Persian)]. Journal of Ardabil University of Medical Sciences. 2010; 10(4):295-302. [Link]
- Sun P, Li K, Wang T, Ji J, Wang Y, Chen KX, et al. Procyanidin C1, a component of cinnamon extracts, is a potential insulin sensitizer that targets adipocytes. Journal of Agricultural and Food chemistry. 2019; 67(32):8839-46. [DOI:10.1021/acs.jafc.9b02932] [PMID]
- Qi L, Hu FB, Hu G. Genes, environment, and interactions in prevention of type 2 diabetes: A focus on physical activity and lifestyle changes. Current Molecular Medicine. 2008; 8(6):519-32. [DOI:10.2174/156652408785747915] [PMID]
- Wei M, Ong L, Smith MT, Ross FB, Schmid K, Hoey AJ, et al. The streptozotocin-diabetic rat as a model of the chronic complications of human diabetes. Heart, Lung and Circulation. 2003; 12(1):44-50. [DOI:10.1046/j.1444-2892.2003.00160.x] [PMID]
- Abedi B, Okhovat E, Banitalebi E. [Comparing the effects of intense sprint and combined aerobic-strength training on serum adiponectin level and insulin resistance among the women with type 2 diabetes (Persian)]. Feyz. 2016; 20(4):352-60. [Link]
- Tashakori Zade M, Mogharnasi M. A study of the effect of 10 weeks of resistance training on HSP70 and insulin resistance in type 2 diabetic women. Journal of Sport Biosciences. 2016; 8(3):341-51. https://jsb.ut.ac.ir/article_59509.html?lang=en
- Bayat M, Alaee M, Akbari A, Sadegh M, Latifi SA, Parastesh M, et al. A comparative study of the antidiabetic effect of two training protocols in streptozotocin-nicotinamide diabetic rats. Hormone Molecular Biology and Clinical Investigation. 2020; 41(2). [DOI:10.1515/hmbci-2019-0046] [PMID]
- Cho Y, Tachibana S, Hazen BC, Moresco JJ, Yates III JR, Kok B, et al. Perm1 regulates CaMKII activation and shapes skeletal muscle responses to endurance exercise training. Molecular metabolism. 2019; 23:88-97. [DOI:10.1016/j.molmet.2019.02.009] [PMID] [PMCID]
- Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. Journal of Diabetes Investigation. 2014; 5(4):349-58. [DOI:10.1111/jdi.12235] [PMID] [PMCID]
- Chae C, Jung S, An S, Park B, Wang S, Cho I, et al. RETRACTED: Treadmill exercise improves cognitive function and facilitates nerve growth factor signaling by activating mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 in the streptozotocin-induced diabetic rat hippocampus. Neuroscience. 2009; 164(4):1665-73. [DOI:10.1016/j.neuroscience.2009.09.075] [PMID]
- Jafari A, Hosseinpourfaizi M, Houshmand M, Ravasi A. Effect of aerobic exercise training on mtDNA deletion in soleus muscle of trained and untrained Wistar rats. British Journal of Sports Medicine. 2005; 39(8):517-20. [DOI:10.1136/bjsm.2004.014068] [PMID] [PMCID]
- Moselhy SS, Junbi HH. Antioxidant properties of ethanolic and aqueous Cinnamon extracts against liver injury in rats. International Journal of Advances in Pharmaceutical Sciences. 2010; 1(2). [Link]
- Fayaz E, Damirchi A, Zebardast N, Babaei P. Cinnamon extract combined with high-intensity endurance training alleviates metabolic syndrome via non-canonical WNT signaling. Nutrition. 2019; 65:173-8. [DOI:10.1016/j.nut.2019.03.009] [PMID]
- Arabmomeni A, Haji Hidari M. [Comparing the effects of three methods, cinnamon supplementation, aerobic exercise and concurrent (aerobic exercise-supplement) on serum glucose, insulin and insulin resistance in type 2 diabetic patients (Persian)]. Medical Journal of Mashhad University of Medical Sciences. 2019; 62(2):1430-9. [DOI:10.22038/MJMS.2019.14119]
- Baharloo S, Taghiyan F, Hedayati M. [Effect of aerobic exercise on glucose, insulin and insulin resistance in subclinical hypothyroidism overweight-obese women (Persian)]. Razi Journal of Medical Sciences. 2014; 21(125):75-84. [Link]
- McCormack SE, McCarthy MA, Harrington SG, Farilla L, Hrovat MI, Systrom DM, et al. Effects of exercise and lifestyle modification on fitness, insulin resistance, skeletal muscle oxidative phosphorylation and intramyocellular lipid content in obese children and adolescents. Pediatric Obesity. 2014; 9(4):281-91. [DOI:10.1111/j.2047-6310.2013.00180.x] [PMID] [PMCID]
- Ramzany N, Gaeini A, Choobineh S, Kordi M, Hedayati M. [Changes of RBP-4 and insulin resistance after 8 weeks of aerobic training in type 2 diabetic rats (Persian)]. Metabolism and Exercise. 2016; 5(2):89-98. [Link]
- Hosseini SE, Shojaei ST, Hosseini SA. [The effects of cinnamon on glycemic indexes and insulin resistance in adult male diabetic rats with streptozotocin. Yafteh. 2015; 16(4):70-8. [Link]
- Zhang W, Xu YC, Guo FJ, Meng Y, Li Ml. Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4 expression in rats with type 2 diabetes mellitus. Chinese Medical Journal. 2008; 121(21):2124-8. [DOI:10.1097/00029330-200811010-00003] [PMID]
- Mahdi A, Kövamees O, Checa A, Wheelock C, Von Heijne M, Alvarsson M, et al. Arginase inhibition improves endothelial function in patients with type 2 diabetes mellitus despite intensive glucose-lowering therapy. Journal of Internal Medicine. 2018; 284(4):388-98. [DOI:10.1111/joim.12785] [PMID]
- Pernow J, Jung C. Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovascular Research. 2013; 98(3):334-43. [DOI:10.1093/cvr/cvt036] [PMID]
- Zhou L, Sun CB, Liu C, Fan Y, Zhu HY, Wu XW, et al. Upregulation of arginase activity contributes to intracellular ROS production induced by high glucose in H9c2 cells. International Journal of Clinical and Experimental Pathology. 2015; 8(3):2728-36. [PMID]
- Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, et al. Diabetes-induced vascular dysfunction involves arginase I. American Journal of Physiology-Heart and Circulatory Physiology. 2012; 302(1):H159-66. [DOI:10.1152/ajpheart.00774.2011] [PMID] [PMCID]
- You H, Gao T, Cooper TK, Morris Jr SM, Awad AS. Arginase inhibition mediates renal tissue protection in diabetic nephropathy by a nitric oxide synthase 3-dependent mechanism. Kidney International. 2013; 84(6):1189-97. [DOI:10.1038/ki.2013.215] [PMID] [PMCID]
- Morris SM, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes. 2011; 60(11):3015-22. [DOI:10.2337/db11-0901] [PMID] [PMCID]
- Semeraro F, Morescalchi F, Cancarini A, Russo A, Rezzola S, Costagliola C. Diabetic retinopathy, a vascular and inflammatory disease: therapeutic implications. Diabetes & Metabolism. 2019; 45(6):517-27. [DOI:10.1016/j.diabet.2019.04.002] [PMID]
- Atawia RT, Toque HA, Meghil MM, Benson TW, Yiew NK, Cutler CW, et al. Role of arginase 2 in systemic metabolic activity and adipose tissue fatty acid metabolism in diet-induced obese mice. International Journal of Molecular Sciences. 2019; 20(6):1462. [DOI:10.3390/ijms20061462] [PMID] [PMCID]
- Jung C, Gonon AT, Sjöquist PO, Lundberg JO, Pernow J. Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovascular Research. 2010; 85(1):147-54. [DOI:10.1093/cvr/cvp303] [PMID]
- Gonon AT, Jung C, Katz A, Westerblad H, Shemyakin A, Sjöquist PO, et al. Local arginase inhibition during early reperfusion mediates cardioprotection via increased nitric oxide production. PLoS One. 2012; 7(7):e42038. [DOI:10.1371/journal.pone.0042038] [PMID] [PMCID]
- Rojas M, Lemtalsi T, Toque HA, Xu Z, Fulton D, Caldwell RW, et al. NOX2-induced activation of arginase and diabetes-induced retinal endothelial cell senescence. Antioxidants. 2017; 6(2):43. [DOI:10.3390/antiox6020043] [PMID] [PMCID]
- Schoene NW, Kelly MA, Polansky MM, Anderson RA. A polyphenol mixture from cinnamon targets P38 MAP kinase-regulated signaling pathways to produce G2/M arrest. The Journal of Nutritional Biochemistry. 2009; 20(8):614-20. [DOI:10.1016/j.jnutbio.2008.06.006] [PMID]
- Chung J, Kim S, Lee HA, Park MH, Kim S, Song YR, et al. Trans-cinnamic aldehyde inhibits Aggregatibacter actinomycetemcomitans-induced inflammation in THP-1-derived macrophages via autophagy activation. Journal of Periodontology. 2018; 89(10):1262-71. [DOI:10.1002/JPER.17-0727] [PMID]
- Grönros J, Jung C, Lundberg JO, Cerrato R, Östenson C-G, Pernow J. Arginase inhibition restores in vivo coronary microvascular function in type 2 diabetic rats. American Journal of Physiology-Heart and Circulatory Physiology. 2011; 300(4):H1174-81. [DOI:10.1152/ajpheart.00560.2010] [PMID]
- Shemyakin A, Kövamees O, Rafnsson A, Böhm F, Svenarud P, Settergren M, et al. Arginase inhibition improves endothelial function in patients with coronary artery disease and type 2 diabetes mellitus. Circulation. 2012; 126(25):2943-50. [DOI:10.1161/CIRCULATIONAHA.112.140335] [PMID]
- Mahdi A, Pernow J, Kövamees O. Arginase inhibition improves endothelial function in an age-dependent manner in healthy elderly humans. Rejuvenation Research. 2019; 22(5):385-9. [DOI:10.1089/rej.2018.2135] [PMID]

