BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://cmja.arakmu.ac.ir/article-1-753-en.html
2- Department of Physiology, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
1. Introduction
Renal toxicity and ototoxicity are considered as the main side effects of the gentamicin [1]. These side effects are associated with the production of Reactive Oxygen Species (ROS). During gentamicin-induced renal toxicity, the tubular epithelial cell necrosis and glomerular damages create ROS. The ROS leads to the contraction of glomerular mesenchymal cells, increases renal vascular resistance, and reduces renal blood flow and glomerular filtration [1]. Thus, the selective aggregation of gentamicin in renal tissues damages various types of cells [2].
Origanum Vulgare (OV) from the mint family is a medicinal plant; the leaves and flower branches of this plant are used medicinally. OV includes several pharmaceutically active compounds, such as linalool, thymol, carvacrol, myrcene, caryophyllene, tannin, glycoside, saponin, and rosaniline acetate [6, 7]. The terpenes compounds of OV, like carvacrol acetate and thymol, have significantly decreased the ROS and nitrogen oxide levels [10]. Moreover, the phenolic compounds of OV have protective effects against ROS and strengthen the body’s antioxidant system [13, 14]. Considering the antioxidant properties of the hydroethanolic extract of OV, the present study aimed to investigate the effect of co-treatment by OV extract on the gentamicin-induced renal toxicity.
2. Materials and Methods
This experimental study was conducted on a sample of 32 adult male Wistar rats with a weight range of 200 to 250 grams. The rats were assigned into four groups: Control, gentamicin, OV+ normal saline, OV+ gentamicin. The control group received no treatment. The gentamicin (100 mg/kg) was intraperitoneally injected to the rats of the gentamicin group, for eight days. Animals in the OV+ normal saline group daily received the gavage of hydroethanolic extract of OV (40 mg/kg) and normal saline (0.5 ml), for eight days. Furthermore, the OV+ gentamicin group received the gavage of hydroethanolic extract of OV (40 mg/kg) and intraperitoneal injection of gentamicin (100 mg/kg). On the ninth day of the experiment, the urine samples were collected, also, the blood pressure was measured from the Caudal artery. Next, the blood sampling was conducted from the abdominal aorta of the animals.
The blood samples were tested for Creatinine (Cr), Blood Urea Nitrogen (BUN), and the sodium and potassium concentrations. Also, the osmolality was measured in blood and urine samples. The observed values were used to Calculate Creatinine Clearance (CCR), absolute excretion of sodium (UNaVo) and potassium (UKVo), and fractional excretion of sodium (FENa) and potassium (FEK) using the suggested equations [17]. On the other hand, the kidneys were delivered to the pathology laboratory; the left and right kidneys were used to determine the tissue damage and measure Malondialdehyde (MDA) with FRAP test, respectively.
The one-way analysis of variance and Tukey test were performed to compare the treatment results’ differences, in SPSS-21. Also, the histological results were analyzed with the Kruskal Wallis and Dunnett tests. The P-value of lower than 0.05 was considered as a significant result in the analysis.
3. Results
Gentamicin treatment increased the levels of Cr, BUN, FENa, FEK, UNaVo, and MDA, also, it reduced the CCR level and the FRAP value of the renal tissue (antioxidant power), and had no significant effect on UKVo. However, OV extract co-treatment decreased the levels of Cr, BUN, FENa, FEK, UNaVo, and MDA, also, it increased the CCR level, the FRAP value, and had no significant effect on UKVo (Table 1).

Thus, gentamicin treatment caused renal toxicity. However, the systolic blood pressure did not differ between the rats with renal toxicity and those without gentamicin treatment.
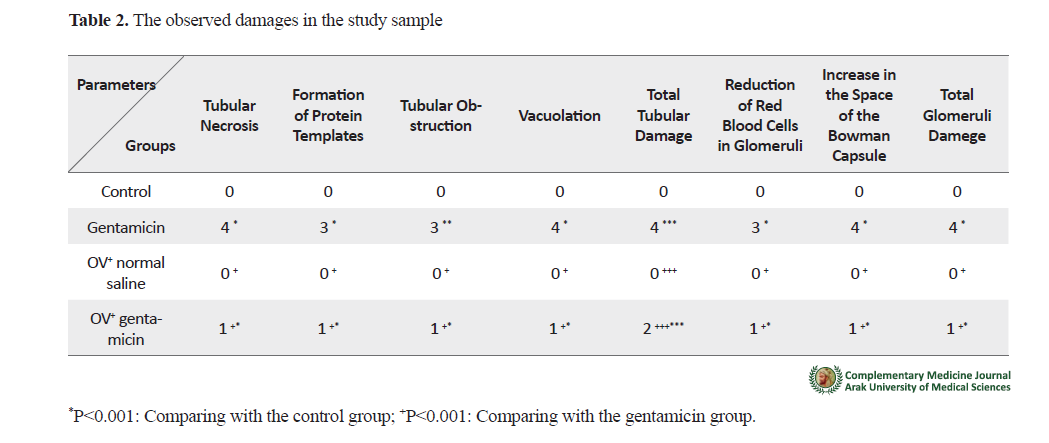
Histological investigation results showed that gentamicin caused severe kidney tissue damage in the renal toxicity group, compared with the control group. The main observed damages include tubular necrosis, increase in the urinary space of the Bowman’s capsule, vacuolation, formation of protein templates, reduction in the number of red blood cells of the glomeruli, and tubular obstruction. Again, the OV extract co-treatment mostly protected the kidney tissue against the observed damages (Table 2).
4. Discussion
In the present study, gentamicin increased the plasma levels of Cr and BUN, also, it reduced the clearance. However, OV extract co-treatment prevented the gentamicin-induced renal toxicity in rats. Also, the result showed that the parameters of oxidative stress and renal excretory function significantly differ between the control and gentamicin groups. Previous studies have shown that phenolic compounds reduce the plasma and urine levels of Cr because these compounds neutralize free radicals resulted from the gentamicin treatment. Thus, the phenolic compounds of hydroethanolic extract of OV can affect the development of renal toxicity due to a simultaneous gentamicin treatment [26, 27]. Besides, our results indicated that the antioxidant compounds of the OV extract can reduce the excretion of sodium and potassium; this occurs owing to the reduction of free radicals and oxidative stress by OV extract [31].
OV extract co-treatment prevented tissue damage in rats with gentamicin-induced renal toxicity. The hydroxyl group in the phenolic compounds of OV extract has regenerative properties and can trap free radicals. Also, the co-treatment with OV extract in rats receiving gentamicin decreased the amount of MDA in kidney tissue and increased the FRAP value. Previous studies have also reported that co-treatment with hydroethanolic extract of OV reduces the oxidative stress and has a protective effect on kidney tissue [25, 26].
5. Conclusion
The findings of this study showed that the antioxidant, anti-inflammatory, and vasodilatory properties of co-treatment with hydroethanolic extract of OV protects rats against gentamicin-induced renal damage. Further studies are needed to identify the active components of the ethanolic extract of OV, investigate the mechanism of their effect on the kidneys, and compare the effects of these compounds. Thus, OV extract could be recommended as a medicinal plant to prevent the renal toxicity of gentamicin.
Ethical Considerations
Compliance with ethical guidelines
The present study was confirmed by the Ethics Committee of the Arak University of Medical Sciences (Ethics Code, IR.ARAKMU.REC.1394284).
Funding
This study is the result of a research project approved by the Student Research Committee of Arak University of Medical Sciences.
Authors' contributions
Conceptualization, methodology, validation, and data analysis: Saeed Hajihashemi; Conducting research and experiments, collecting data, and reviewing the sources of drafting, Razieh Rajabi and Atefeh Ghiasabadi Farahani.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the Vice-Chancellor for Research and Information Technology of Arak University of Medical Sciences for their financial support.
References
1.Randjelović P, Veljković S, Stojiljković N, Sokolović D, Ilić I. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI Journal. 2017; 16:388-99. [DOI:10.17179/excli2017-165] [PMID] [PMCID]
2.Quiros Y, Vicente-Vicente L, Morales AI, López-Novoa JM, López-Hernández FJ. An integrative overview on the mechanisms underlying the renal tubular cytotoxicity of gentamicin. Toxicological Sciences. 2011; 119(2):245-56. [DOI:10.1093/toxsci/kfq267] [PMID]
3.Nagai J, Takano M. Entry of aminoglycosides into renal tubular epithelial cells via endocytosis-dependent and endocytosis-independent pathways. Biochemical Pharmacology. 2014; 90(4):331-7. [DOI:10.1016/j.bcp.2014.05.018] [PMID]
4.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biology and Medicine. 2010; 48(6):749-62. [DOI:10.1016/j.freeradbiomed.2009.12.022] [PMID] [PMCID]
5.Morshedloo MR, Ahmadi H, Pirali Hamedani M, Yazdani D. [An over review to Origanum vulgare L. and its pharmacological properties (Persian)]. Journal of Medicinal Plants. 2018; 17(68):15-31. http://jmp.ir/article-1-2289-en.html
6.Oniga I, Pușcaș C, Silaghi-Dumitrescu R, Olah NK, Sevastre B, Marica R, et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules. 2018; 23(8):2077. [DOI:10.3390/molecules23082077] [PMID] [PMCID]
7.Azizi H, Keshavarzi M. Ethnobotanical study of medicinal plants of Sardasht, Western Azerbaijan, Iran. Journal of Herbal Drugs. 2015; 6(2):113-9. http://jhd.iaushk.ac.ir/article_643571.html
8.Mombeini T, Mombeini M, Aghayi M. [Evaluation of pharmacological effects of Origanum genus (Origanum spp.) (Persian)]. Journal of Medicinal Plants. 2009; 8(29):18-35. http://jmp.ir/article-1-380-en.html
9.Ocaña-Fuentes A, Arranz-Gutiérrez E, Señorans FJ, Reglero G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: Anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food and Chemical Toxicology. 2010; 48(6):1568-75. [DOI:10.1016/j.fct.2010.03.026] [PMID]
10.Zhang XL, Guo YS, Wang CH, Li GQ, Xu JJ, Chung HY, et al. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chemistry. 2014; 152:300-6. [DOI:10.1016/j.foodchem.2013.11.153] [PMID]
11.Mueller M, Lukas B, Novak J, Simoncini T, Genazzani AR, Jungbauer A. Oregano: A source for peroxisome proliferator-activated receptor γ antagonists. Journal of Agricultural and food Chemistry. 2008; 56(24):11621-30. [DOI:10.1021/jf802298w] [PMID]
12.Leyva-López N, Nair V, Bang WY, Cisneros-Zevallos L, Basilio Heredia J. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. Journal of Ethnopharmacology. 2016; 187:302-12. [DOI:10.1016/j.jep.2016.04.051] [PMID]
13.Şahin F, Güllüce M, Daferera D, Sökmen A, Sökmen M, Polissiou M, et al. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp. vulgare in the Eastern Anatolia region of Turkey. Food Control. 2004; 15(7):549-57. [DOI:10.1016/j.foodcont.2003.08.009]
14.Liu H, Zheng A, Liu H, Yu H, Wu X, Xiao C, et al. Identification of three novel polyphenolic compounds, origanine A-C, with unique skeleton from Origanum vulgare L. using the hyphenated LC-DAD-SPE-NMR/MS methods. Journal of Agricultural and Food Chemistry. 2012; 60(1):129-35. [DOI:10.1021/jf204406u] [PMID]
15.Hajihashemi S, Rajabi R, Ghiasabadi Farahani A. [The oral post-treatment effect of hydroethanolic extract of Origanum vulgare on acute kidney injury caused by gentamicin in rats (Persian-English)]. Journal of Arak University of Medical Sciences. 2019; 22(5):18-31. [DOI:10.32598/JAMS.22.5.18]
16.Hajihashemi S, Jafarian T, Ahmadi M, Rahbari A, Ghanbari F. Ameliorative effects of Zataria multiflora hydro-alcoholic extract on gentamicin induced nephrotoxicity in rats. Drug Research. 2018; 68(07):387-94. [DOI:10.1055/s-0043-124968] [PMID]
17.Ahmadi F, Hajihashemi S, Rahbari A, Ghanbari F. Effects of nitroglycerine on renal ischemia-reperfusion injury in adult male rats. Drug Research. 2019; 69(11):612-20. [DOI:10.1055/a-0958-1987] [PMID]
18.Sharifi-Rigi A, Heidarian E. Therapeutic potential of Origanum vulgare leaf hydroethanolic extract against renal oxidative stress and nephrotoxicity induced by paraquat in rats. Avicenna Journal of Phytomedicine. 2019; 9(6):563-73. [DOI:10.22038/AJP.2019.13466] [PMID] [PMCID]
19.Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996; 239(1):70-6. [DOI:10.1006/abio.1996.0292] [PMID]
20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979; 95(2):351-8. [DOI:10.1016/0003-2697(79)90738-3]
21.Al-Shabanah OA, Aleisa AM, Al-Yahya AA, Al-Rejaie SS, Bakheet SA, Fatani AG, et al. Increased urinary losses of carnitine and decreased intramitochondrial coenzyme A in gentamicin-induced acute renal failure in rats. Nephrology Dialysis Transplantation. 2010; 25(1):69-76. [DOI:10.1093/ndt/gfp457] [PMID]
22.Savin V, Karniski L, Cuppage F, Hodges G, Chonko A. Effect of gentamicin on isolated glomeruli and proximal tubules of the rabbit. Laboratory Investigation. 1985; 52(1):93-102. [PMID]
23.Karahan İ, Ateşşahin A, Yılmaz S, Çeribaşı AO, Sakin F. Protective effect of lycopene on gentamicin-induced oxidative stress and nephrotoxicity in rats. Toxicology. 2005; 215(3):198-204. [DOI:10.1016/j.tox.2005.07.007] [PMID]
24.Kaurinovic B, Popovic M, Vlaisavljevic S, Trivic S. Antioxidant capacity of Ocimum basilicum L. and Origanum vulgare L. extracts. Molecules. 2011; 16(9):7401-14. [DOI:10.3390/molecules16097401] [PMID] [PMCID]
25.Kaledaite R, Bernatoniene J, Majiene D, Dvorackova K, Masteikova R, Muselik J, et al. Investigation of antiradical activity of Salvia officinalis L., Urtica dioica L., and Thymus vulgaris L. extracts as potential candidates for a complex therapeutic preparation. Journal of Medicinal Plants Research. 2011; 5(25):6090-6. https://academicjournals.org/journal/JMPR/article-stat/4C5BDC821230
26.Ali NAM, Saeed SZ. Nephro-protective effect of Punica granatum in gentamicin-induced nephrotoxicity in rats. Medical Journal of Babylon. 2012; 9(1):220-8. https://www.iasj.net/iasj?func=fulltext&aId=35508
27.Gowrisri M, Sarita K, Vrushabendra Swamy BM, Archana Swamy P, Vishwanath KM. Anti-oxidant and nephroprotective activities of Cassia occidentalis leaf extract against gentamicin induced nephrotoxicity in rats. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2012; 3(3):684-94. https://www.researchgate.net/publication/286882273
28.Bae WK, Lee JU, Park JW, Bae EH, Ma SK, Kim SH, et al. Decreased expression of Na+/K+-ATPase, NHE3, NBC1, AQP1 and OAT in gentamicin-induced nephropathy. The Korean Journal of Physiology & Pharmacology. 2008; 12(6):331-6. [DOI:10.4196/kjpp.2008.12.6.331] [PMID] [PMCID]
29.Williams PD, Trimble ME, Crespo L, Holohan PD, Freedman JC, Ross CR. Inhibition of renal Na+, K+-adenosine triphosphatase by gentamicin. Journal of Pharmacology and Experimental Therapeutics. 1984; 231(2):248-53. [PMID]
30.Beltrán JMG, Espinosa C, Guardiola FA, Ángeles Esteban M. In vitro effects of Origanum vulgare leaf extracts on gilthead seabream (Sparus aurata L.) leucocytes, cytotoxic, bactericidal and antioxidant activities. Fish & Shellfish Immunology. 2018; 79:1-10. [DOI:10.1016/j.fsi.2018.05.005] [PMID]
31.Han F, Ma GQ, Yang M, Yan L, Xiong W, Shu JC, et al. Chemical composition and antioxidant activities of essential oils from different parts of the oregano. Journal of Zhejiang University-Science B. 2017; 18(1):79-84. [DOI:10.1631/jzus.B1600377] [PMID] [PMCID]
32.Hajihashemi S, Hamidizad Z, Rahbari A, Ghanbari F, Aghaee Motealeghi Z. Effects of Cobalamin (Vitamin B12) on gentamicin induced nephrotoxicity in rat. Drug Research. 2017; 67(12):710-8. [DOI:10.1055/s-0043-117418] [PMID]
33.Neugarten J, Aynedjian HS, Bank N. Role of tubular obstruction in acute renal failure due to gentamicin. Kidney International. 1983; 24:330-5. [DOI:10.1038/ki.1983.162] [PMID]
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








